Vapour Pressure
Vapour pressure is the pressure measured when a system reaches equilibrium
Vapour pressure is dependent on temperature and the type of liquid
Vapour pressure of water
| 0 | 0.6113 | 4.5851 | 0.0060 |
| 5 | 0.8726 | 6.5450 | 0.0086 |
| 10 | 1.2281 | 9.2115 | 0.0121 |
| 15 | 1.7056 | 12.7931 | 0.0168 |
| 20 | 2.3388 | 17.5424 | 0.0231 |
| 25 | 3.1690 | 23.7695 | 0.0313 |
| 30 | 4.2455 | 31.8439 | 0.0419 |
| 35 | 5.6267 | 42.2037 | 0.0555 |
| 40 | 7.3814 | 55.3651 | 0.0728 |
| 45 | 9.5898 | 71.9294 | 0.0946 |
| 50 | 12.3440 | 92.5876 | 0.1218 |
| 55 | 15.7520 | 118.1497 | 0.1555 |
| 60 | 19.9320 | 149.5023 | 0.1967 |
| 65 | 25.0220 | 187.6804 | 0.2469 |
| 70 | 31.1760 | 233.8392 | 0.3077 |
| 75 | 38.5630 | 289.2463 | 0.3806 |
| 80 | 47.3730 | 355.3267 | 0.4675 |
| 85 | 57.8150 | 433.6482 | 0.5706 |
| 90 | 70.1170 | 525.9208 | 0.6920 |
| 95 | 84.5290 | 634.0196 | 0.8342 |
| 100 | 101.3200 | 759.9625 | 1.0000 |
As a result of a chemical reaction,
The total volume of the product is
solution
Assume liquid-vapour equilibrium.
From the table at
Now to find the mass of vapour and liquid:
Note that this assumes that the volume occupied by the vapour phase is equivalent to the volume of the container. Since the liquid phase occupies at most
Note that when starting with this assumption, and calculate a mass larger than the total mass, you conclude that the system contains only vapour at a pressure lower than the vapour pressure.
Is the sample only in the liquid phase?
The density of water is
Is the sample only in the vapour phase?
Determine the pressure of the vapour assuming that all
Since this pressure is greater than the vapour pressure of water at
Note that if the pressure were less than the vapour pressure, the system would contain only vapour and we would be finished
Volatility
Weak intermolecular forces -> more likely to evaporate -> high vapour pressure (volatile)
Strong intermolecular forces -> less likely to evaporate -> low vapour pressure (non-volatile)
Evaporation vs Boiling
Evaporation:
- Occurs on surface
- Occurs at any temperature
- Some molecules at the surface have enough energy to overcome intermolecular forces
Boiling:
- Occurs throughout the liquid
- Occurs at a specific temperature known as the boiling point
- Vapour pressure equals surrounding atmospheric pressure
Boiling Point
The temperature at which the vapour pressure is equal to the external pressure
Normal Boiling Point
The temperature at which the vapour pressure is equal to
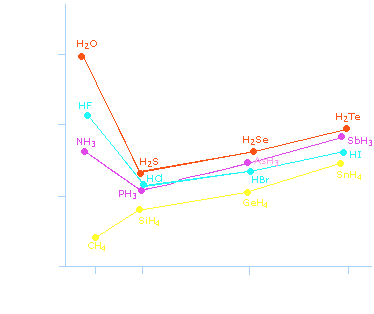
Why is
Rank the following from highest to lowest normal boiling point:
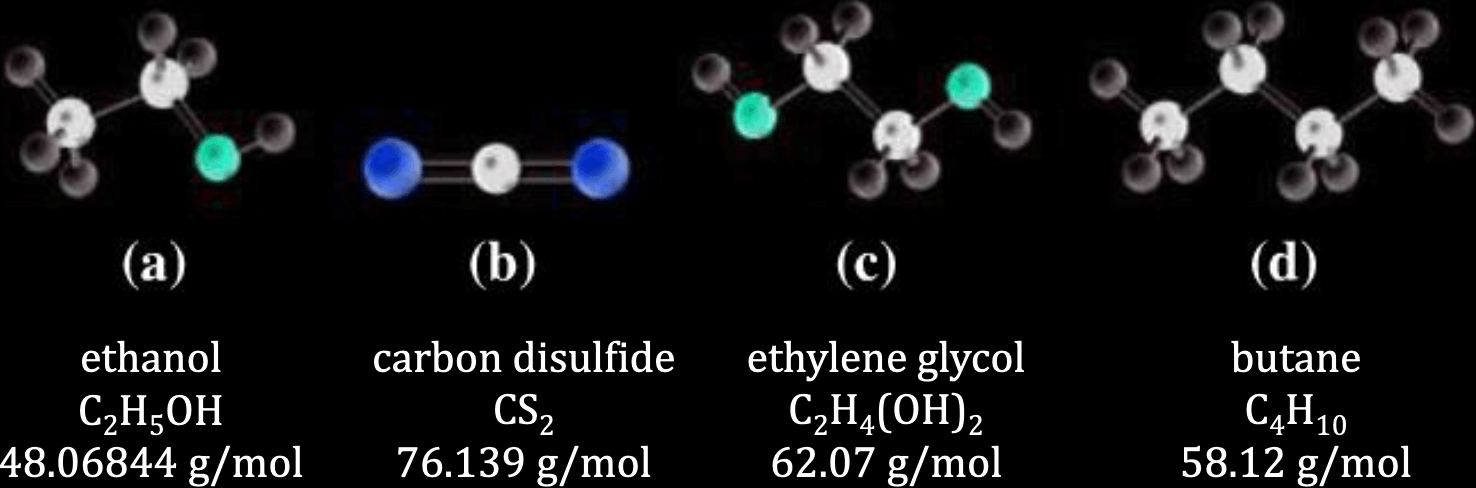
solution
- Ethylene glycol, 2 hydrogen bonds (
) - Ethanol, 1 hydrogen bond (
) - Carbon disulphide, higher molar mass = stronger LDF (
) - Butane, lower molar mass = weaker LDF (
)
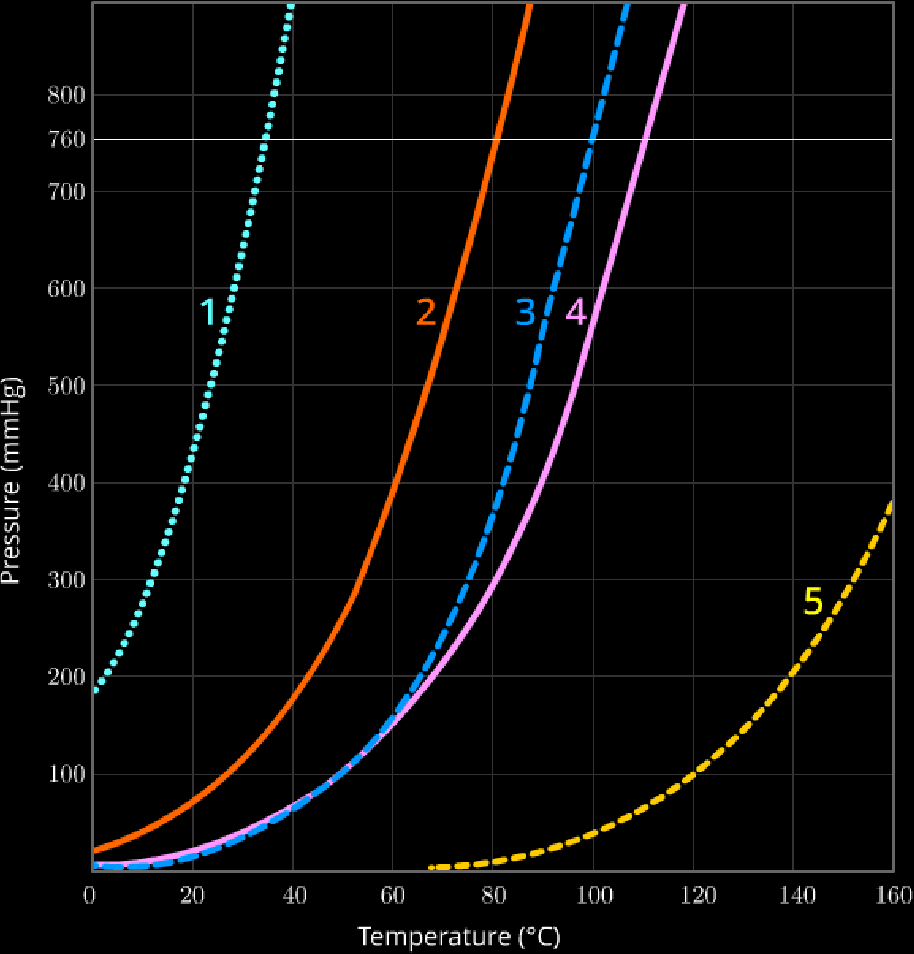
Clausius-Clapeyron Equation
- Diethyl ether,
- Benzene,
- Water,
- Toluene,
- Aniline,
These kinda look like exponential functions,
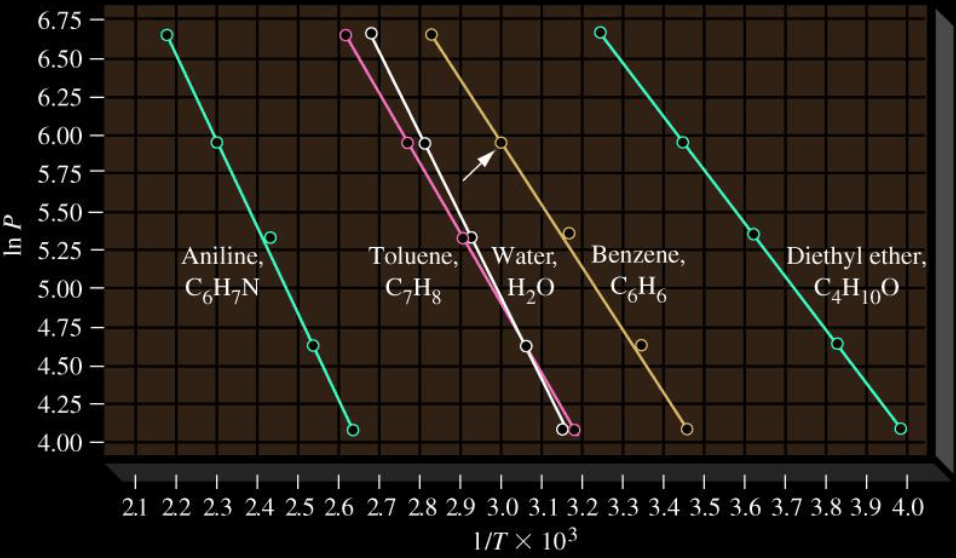
From
We can get rid of B by taking the difference between two points:
Note that we assume
Today's weather report states: "The temperature is
Useful information:
solution
Approximate the vapour pressure of water at
then using Dalton's law: